

We are here to provide personalized support to your patients receiving COSENTYX®
With 8+ years of experience, the XPOSE® Patient Support Program is here for you. The Program aims to deliver a high-quality service to you, and seamless onboarding for patients.
You can count on XPOSE® to be with you and your patients throughout their treatment journey.

XPOSE® Enrollment Form
XPOSE® Enrollment Form

Dedicated Field Case Manager to customize your experience

Taking care of COSENTYX® patients1
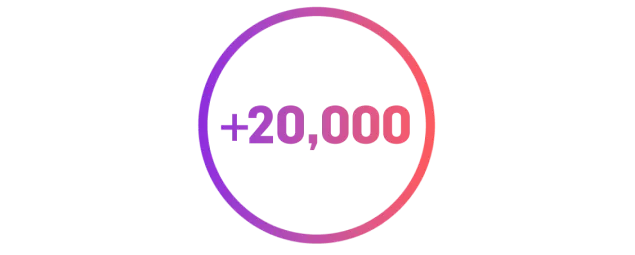
Patients enrolled in XPOSE®1


Now available: AvidityHealth™ for PSP enrollment
Novartis, in partnership with our XPOSE® PSP provider Innomar Strategies, are happy to provide healthcare providers (HCPs) with access to AvidityHealth to facilitate the XPOSE® enrollment process for you and your patients.
AvidityHealth was designed to seamlessly integrate the enrollment into the healthcare provider’s workflow, via their EMRs, allowing them to enroll patients and check on their status throughout the process. HCPs can access built-in features like pre-populating patient data, which will help ensure the enrollment details are fully accurate. AvidityHealth will connect your computer directly to the XPOSE® database.
AvidityHealth delivers:
Connectivity for physician and the XPOSE® program
Both EMR and web solution options, making this solution available to all physicians and their staff
Web solution allows for status updates on all your COSENTYX® patients at all times


XPOSE® is here for you and your patients
If you would like to enroll your patients without the help of the AvidityHealth™ Enrollment Service, simply access the enrollment form below.

XPOSE® Enrollment Form
XPOSE® Enrollment Form
HCP Quotes




COSENTYX® is indicated for the treatment of:
Moderate to severe plaque psoriasis in adult patients who are candidates for systemic therapy or phototherapy.
Moderate to severe plaque psoriasis in pediatric patients 6 years and older who are candidates for systemic therapy or phototherapy.
Active psoriatic arthritis in adult patients when the response to previous disease-modifying anti-rheumatic drug (DMARD) therapy has been inadequate. COSENTYX® can be used alone or in combination with methotrexate.
Active ankylosing spondylitis in adult patients who have responded inadequately to conventional therapy.
Active non-radiographic axial spondyloarthritis with objective signs of inflammation as indicated by elevated C-reactive protein (CRP) and/or magnetic resonance imaging (MRI) evidence in adults who have responded inadequately, or are intolerant to nonsteroidal anti-inflammatory drugs (NSAIDs).
Juvenile idiopathic arthritis categories:
Active enthesitis-related arthritis in patients 6 years and older whose disease has responded inadequately to, or who cannot tolerate, conventional therapy.
Active juvenile psoriatic arthritis in patients 6 years and older whose disease has responded inadequately to, or who cannot tolerate, conventional therapy.
Moderate to severe hidradenitis suppurativa (acne inversa) in adult patients who have responded inadequately to conventional systemic hidradenitis suppurativa therapy.
Consult the Product Monograph at www.novartis.ca/CosentyxMonograph for important information about:
Relevant warnings and precautions regarding infections, inflammatory bowel disease, hypersensitivity reactions, latex-sensitive patients, eczematous eruptions, immunizations, pregnancy and breastfeeding.
Conditions of clinical use, adverse reactions, drug interactions and dosing instructions.
The Product Monograph is also available through our medical department. Call us at 1-800-363-8883.
Reference
Data on file. Novartis Pharmaceuticals Canada Inc.
COSENTYX and SensoReady are registered trademarks.
Product Monograph available on request
431035E
© Novartis Pharmaceuticals Canada Inc. January 2025



