

The recommended dose is based on body weight. For patients weighing <50 kg the dose is 75 mg. For patients weighing ≥50 kg the dose is 150 mg.
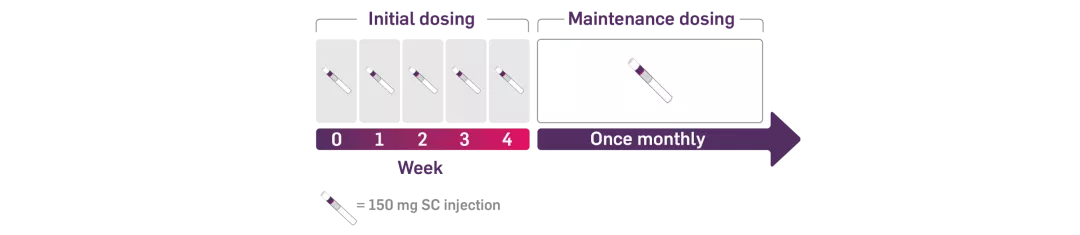
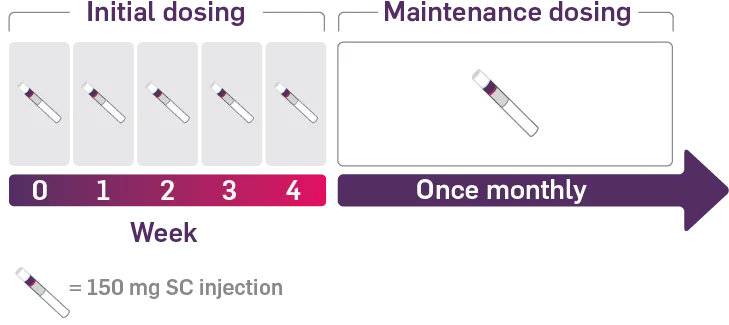
COSENTYX® is administered by subcutaneous injection with initial dosing at Weeks 0, 1, 2, 3, and 4 followed by monthly maintenance dosing.
Each 75 mg dose is given as one subcutaneous injection of 75 mg; each 150 mg dose is given as one subcutaneous injection of 150 mg.
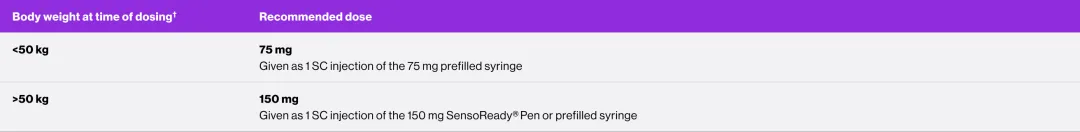
† 50 kg is equal to 110.23 lbs.
Please consult Product Monograph for complete dosing and administration information.
COSENTYX® is indicated for the treatment of:
Moderate to severe plaque psoriasis in adult patients who are candidates for systemic therapy or phototherapy.
Moderate to severe plaque psoriasis in pediatric patients 6 years and older who are candidates for systemic therapy or phototherapy.
Active psoriatic arthritis in adult patients when the response to previous disease-modifying anti-rheumatic drug (DMARD) therapy has been inadequate. COSENTYX® can be used alone or in combination with methotrexate.
Active ankylosing spondylitis in adult patients who have responded inadequately to conventional therapy.
Active non-radiographic axial spondyloarthritis with objective signs of inflammation as indicated by elevated C-reactive protein (CRP) and/or magnetic resonance imaging (MRI) evidence in adults who have responded inadequately, or are intolerant to nonsteroidal anti-inflammatory drugs (NSAIDs).
Juvenile idiopathic arthritis categories:
Active enthesitis-related arthritis in patients 6 years and older whose disease has responded inadequately to, or who cannot tolerate, conventional therapy.
Active juvenile psoriatic arthritis in patients 6 years and older whose disease has responded inadequately to, or who cannot tolerate, conventional therapy.
Moderate to severe hidradenitis suppurativa (acne inversa) in adult patients who have responded inadequately to conventional systemic hidradenitis suppurativa therapy.
Consult the Product Monograph at www.novartis.ca/CosentyxMonograph for important information relating to adverse reactions, drug interactions and dosing information which has not been discussed in this piece. The Product Monograph is also available by calling 1-800-363-8883.
Reference
COSENTYX® Product Monograph. Novartis Pharmaceuticals Canada Inc.
COSENTYX and SensoReady are registered trademarks.
Product Monograph available on request
431035E
© Novartis Pharmaceuticals Canada Inc. January 2025



