
Store between 15°C to 25°C. Do not freeze.1§
LEQVIO® should be administered as soon as possible and patient’s original dosing schedule should be maintained.
A new dosing schedule should begin.
LEQVIO® should be administered as soon as possible, again at 3 months, followed by every 6 months.
LEQVIO® Product Monograph. Novartis Pharmaceuticals Canada Inc.
Data On File. First siRNA PCSK9i. Novartis Pharmaceuticals Inc., 2023.
Data On File. Needle Size. Novartis Pharmaceuticals Inc., 2023.
Non-familial hypercholesterolemia with ASCVD, or
Heterozygous familial hypercholesterolemia (HeFH).
as an adjunct to diet and statin therapy, with or without other lipid-lowering therapies, in patients who require additional lowering of LDL-C
as an adjunct to diet, alone or in combination with non-statin lipid-lowering therapies, in patients for whom a statin is contraindicated
as an adjunct to diet and statin therapy, with or without other lipid-lowering therapies
as an adjunct to diet, as monotherapy or in combination with other non-statin lipid-modifying therapies, in patients for whom a statin is contraindicated.
LEQVIO® Product Monograph. Novartis Pharmaceuticals Canada Inc.
Data On File. First siRNA PCSK9i. Novartis Pharmaceuticals Inc., 2023.
Repatha® (evolocumab injection) Product Monograph. September 2023.
Praluent® (alirocumab injection) Product Monograph. May 2024.
LEQVIO® Product Monograph. Novartis Pharmaceuticals Canada Inc.
Data On File. First siRNA PCSK9i. Novartis Pharmaceuticals Inc., 2023.
Repatha® (evolocumab injection) Product Monograph. September 2023.
Praluent® (alirocumab injection) Product Monograph. May 2024.
LEQVIO® can be dosed twice per year and is administered by a healthcare provider
Dosing Regimen
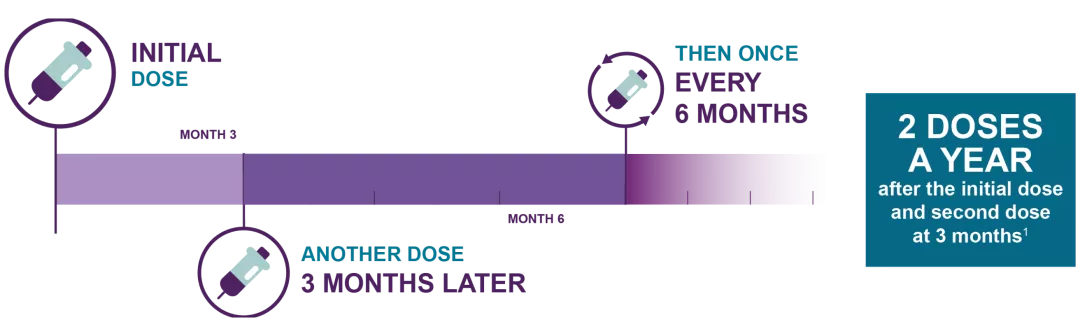
The recommended dose of LEQVIO® is 284 mg administered by a healthcare provider as a single subcutaneous injection: initially, again at 3 months, and then once every 6 months.1
Additional Administration Information
Missed Dose
If a dose of LEQVIO® is missed by:1
<3 months | ≥3 months |
|---|---|
Product Information
References
LEQVIO® is a registered trademark.
© Novartis Pharmaceuticals Canada Inc.
February/2025 - 425307E

Related content
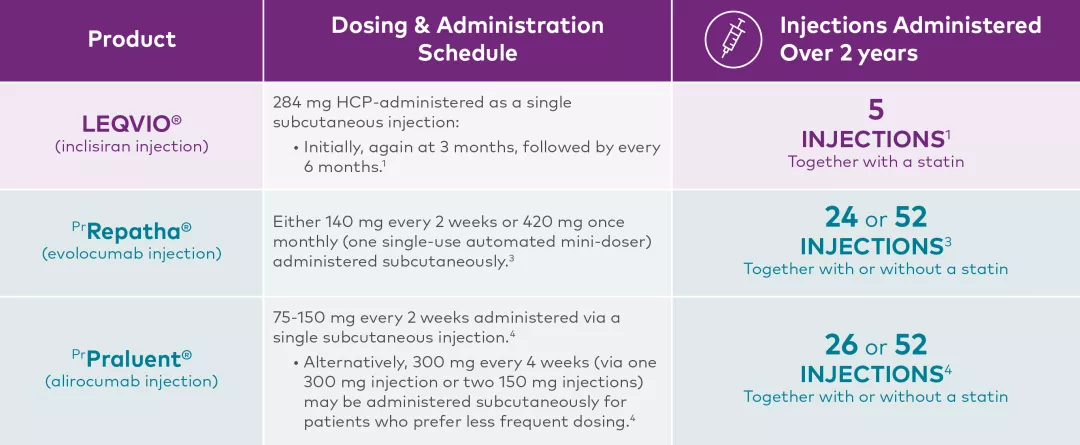
Dosing Regimens for PCSK9 Inhibitors1,3,4†‡
Product | Dosing & Administration Schedule |
LEQVIO® | 284 mg HCP-administered as a single subcutaneous injection: |
LEQVIO® is indicated as an adjunct to lifestyle changes, including diet, to further reduce LDL-C level in adults with the following conditions who are on a maximally tolerated dose of a statin, with or without other LDL-C-lowering therapies: The effect of LEQVIO® on cardiovascular morbidity and mortality has not been determined. | |
PrRepatha® | Either 140 mg every 2 weeks or 420 mg once monthly (one single-use automated mini-doser) administered subcutaneously.3 |
Prevention of Cardiovascular Events: Repatha® is indicated as an adjunct to diet and standard of care therapy (including moderate- to high-intensity statin therapy alone or in combination with other lipid-lowering therapy), to reduce the risk of MI, stroke, and coronary revascularization in adult patients with ASCVD by further lowering low-density lipoprotein cholesterol (LDL-C) levels.3 Primary Hyperlipidemia (including HeFH): Repatha® is indicated for the reduction of elevated LDL-C in adult patients with primary hyperlipidemia (including HeFH):3 Pediatric Patients with Heterozygous Familial Hypercholesterolemia: | |
PrPraluent®
| 75-150 mg every 2 weeks administered via a single subcutaneous injection.4 |
Prevention of Cardiovascular Events: Praluent® is indicated in combination with a maximum tolerated dose of a statin, with or without other lipid-lowering therapies, to reduce the risk of MI, ischemic stroke, and unstable angina requiring hospitalization in adults with established CVD.4 | |
References
LEQVIO® is a registered trademark.
© Novartis Pharmaceuticals Canada Inc.
February/2025 - 425307E

Related content
Comparative Dosing Chart
Know the Dosing Regimens for PCSK9 Inhibitors1,3,4†‡
Obtain this Comparative Dosing Chart for your clinic
References
LEQVIO® is a registered trademark.
© Novartis Pharmaceuticals Canada Inc.
February/2025 - 425307E