
United States
Non-familial hypercholesterolemia
With ASCVD
LEQVIO® Product Monograph. Novartis Pharmaceuticals Canada Inc.
Data on File. First siRNA PCSK9i. Novartis Pharmaceuticals Inc., 2023.
Ray KK, Wright RS, Kallend D, et al. Two Phase 3 Trials of Inclisiran in Patients with Elevated LDL Cholesterol. N Engl J Med. 2020;382(16):1507-1519.
Ray KK, Wright RS, Kallend D, et al. Two Phase 3 Trials of Inclisiran in Patients with Elevated LDL Cholesterol. N Engl J Med. 2020;382(16_Suppl):1-32.
International
HeFH
Diagnosis made via genotyping or clinical criteria (Simon Broome or WHO/Dutch Lipid Network criteria)
LEQVIO® Product Monograph. Novartis Pharmaceuticals Canada Inc.
Data on File. First siRNA PCSK9i. Novartis Pharmaceuticals Inc., 2023.
Raal FJ, Kallend D, Ray KK, et al. Inclisiran for the Treatment of Heterozygous Familial Hypercholesterolemia. N Engl J Med. 2020;382(16):1520-1530.
Coprimary Endpoints
In ORION-10, LEQVIO® significantly reduced LDL-C vs. placebo in patients who have non-familial hypercholesterolemia with ASCVD1,3†‡
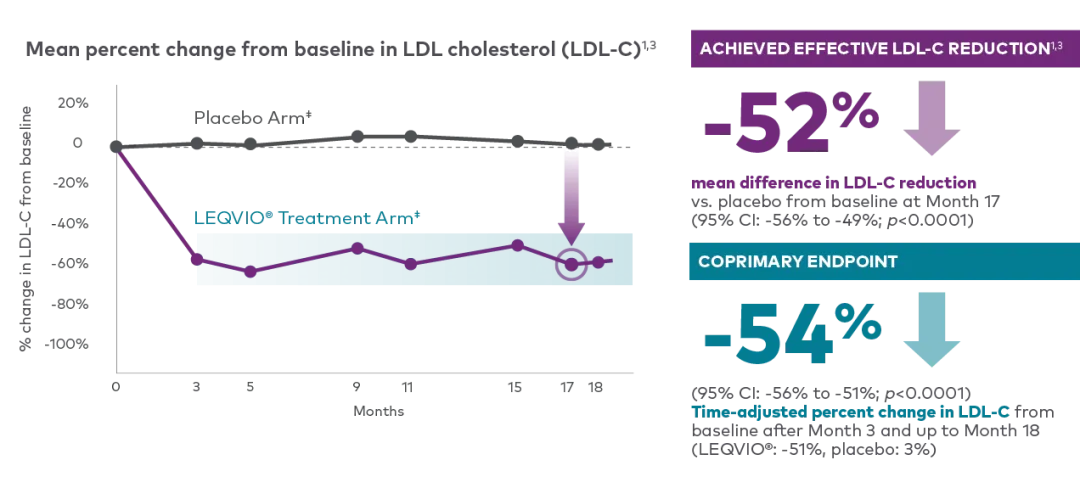
Adapted from the LEQVIO® Product Monograph.
LEQVIO® was also studied in the ORION-11 (N=1,617) clinical trial in a mixed population (patients who had non-familial hypercholesterolemia with ASCVD and/or ASCVD risk equivalent patients).1
Note: LEQVIO® is not indicated for the treatment of patients with ASCVD risk equivalents.
ASCVD=atherosclerotic cardiovascular disease; LDL-C=low-density lipoprotein cholesterol.
† In the ORION-10 (N=1,561) and ORION-11 (N=1,617) clinical trials, patients who received 284 mg of LEQVIO® (inclisiran injection) administered subcutaneously at baseline, 3 months, and every subsequent 6 months exhibited an average between-group difference in LDL-C reduction of 52% (95% CI: -55.7%, -48.8; p<0.001) and -49.9% (95% CI: -53.1, -46.6; p<0.0001) when compared to placebo, respectively. The reduction in LDL-C was maintained across each 6-month dosing interval up to trial Day 510.1
‡ Patients in each study arm in the ORION-10 clinical trial were receiving a maximally tolerated dose of statin, with or without other lipid-modifying therapy, such as ezetimibe.
Other Secondary Outcomes
At Day 510 in ORION-10:
Proportion who achieved an LDL-C <1.8 mmol/L (70 mg/dL):1
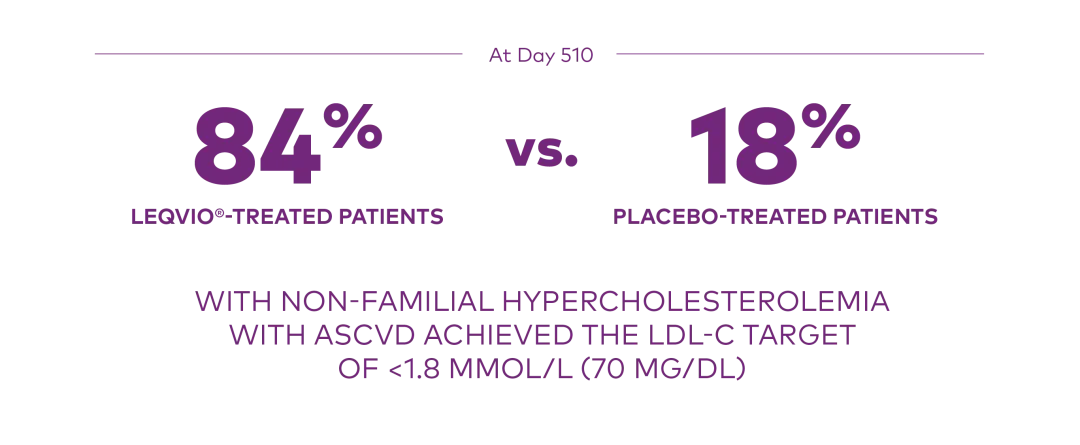
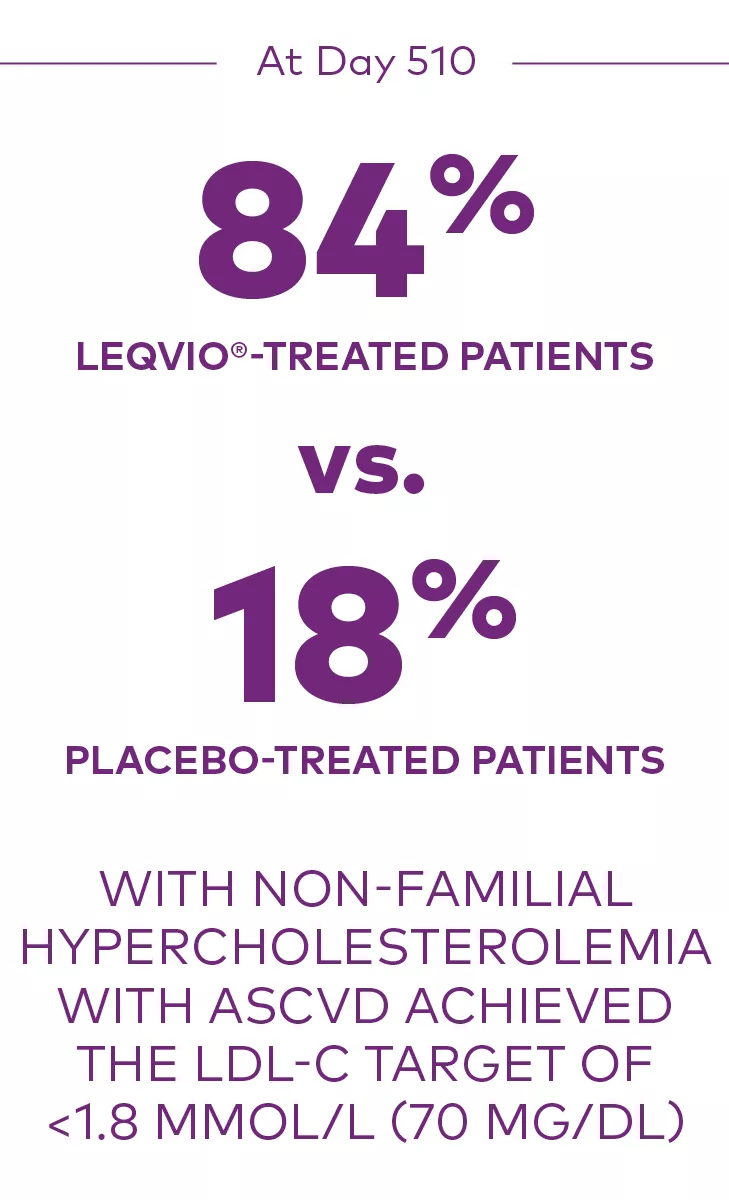
ASCVD=atherosclerotic cardiovascular disease; LDL-C=low-density lipoprotein cholesterol.
Trial Design
ORION-101,3,4
Features | Multicentre, double-blind, randomized (1:1), placebo-controlled |
Location | |
Population |
Treatment Arms | LEQVIO® 284 mg SC (n=781) |
Coprimary Endpoints | Percent change in LDL-C from baseline to Day 510
|
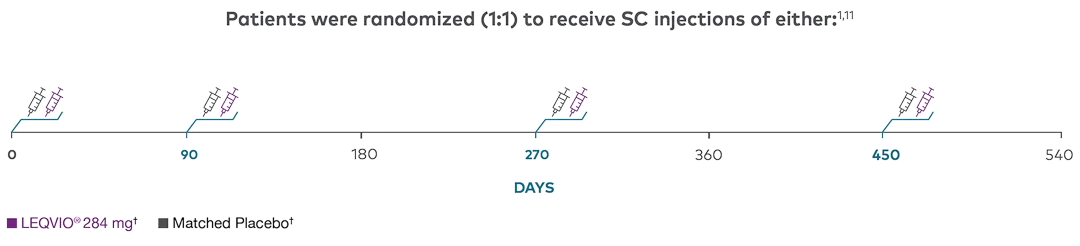
ASCVD=atherosclerotic cardiovascular disease; LDL-C=low-density lipoprotein cholesterol; SC=subcutaneous.
† Patients in each study arm in the ORION-10 phase 3 clinical trials were receiving a maximally tolerated dose of a statin, with or without other lipid-modifying therapy, such as ezetimibe.1,3
References
LEQVIO® is a registered trademark.
© Novartis Pharmaceuticals Canada Inc.
February/2025 - 425307E

Related content
Coprimary Endpoints
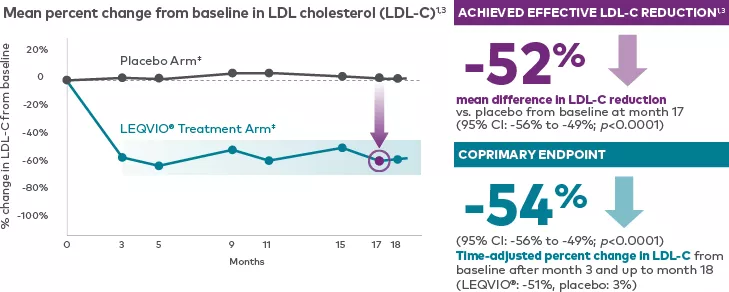
In ORION-9, LEQVIO® significantly reduced LDL-C vs. placebo in patients with HeFH1,3†‡
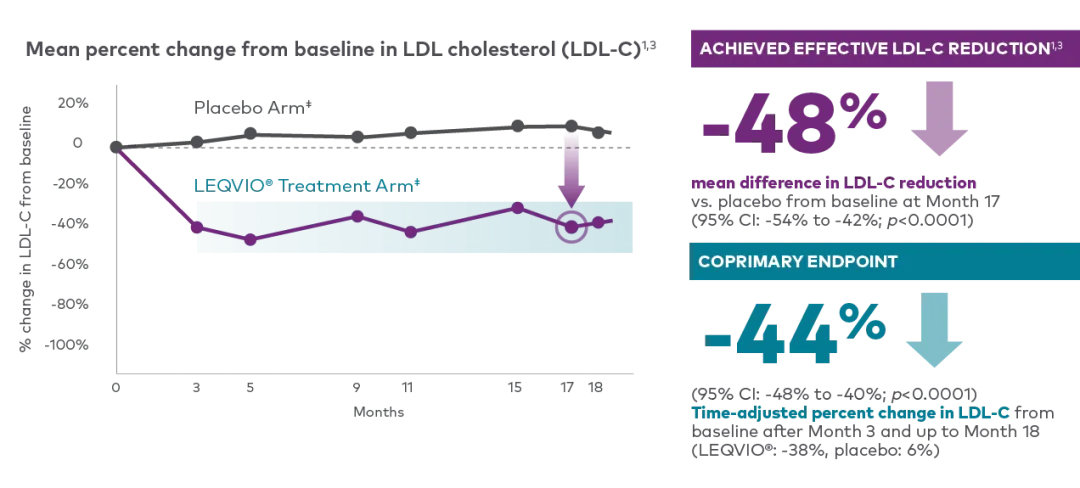

Adapted from the LEQVIO® Product Monograph.
HeFH=heterozygous familial hypercholesterolemia; LDL-C=low density lipoprotein cholesterol.
† In the ORION-9 clinical trial (N=482), patients who received 284 mg of LEQVIO® (inclisiran injection) administered subcutaneously at baseline, 3 months, and every subsequent 6 months exhibited an average between-group difference in LDL-C reduction of 47.9% (95% CI: -53.5%, -42.3%; p<0.0001) when compared to placebo. The reduction in LDL-C was maintained across each 6-month dosing interval up to trial Day 510.1
‡ Patients in the ORION-9 clinical trial were on a maximally tolerated dose of a statin, with or without other lipid-lowering therapies, such as ezetimibe.1
Other Secondary Outcomes
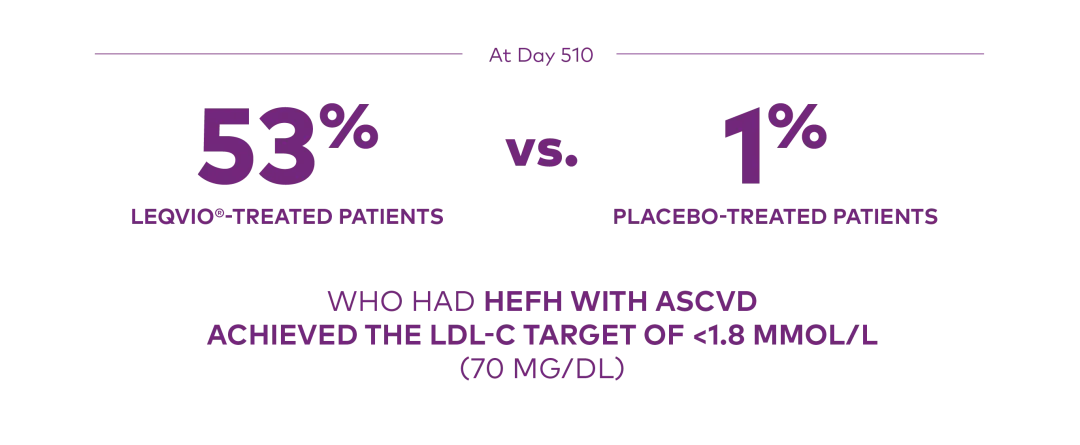
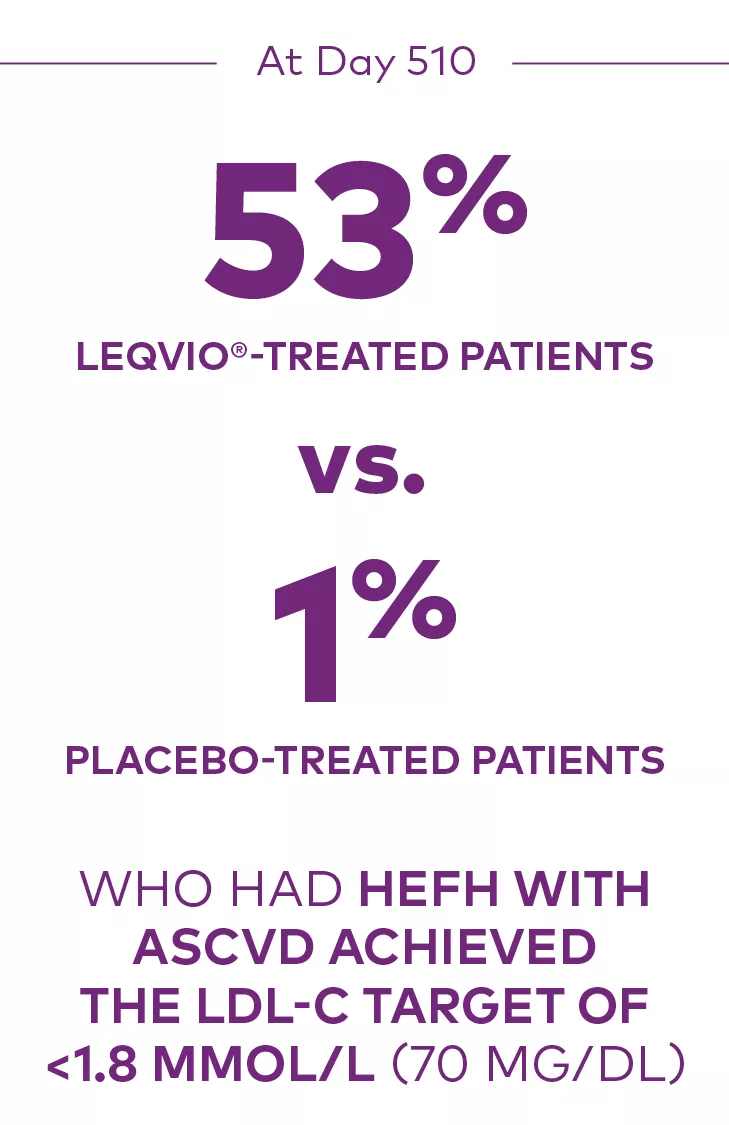
At Day 510 in ORION-9:
Proportion who achieved an LDL-C <1.8 mmol/L (70 mg/dL):1
Proportion who achieved an LDL-C <2.6 mmol/L (100 mg/dL):1
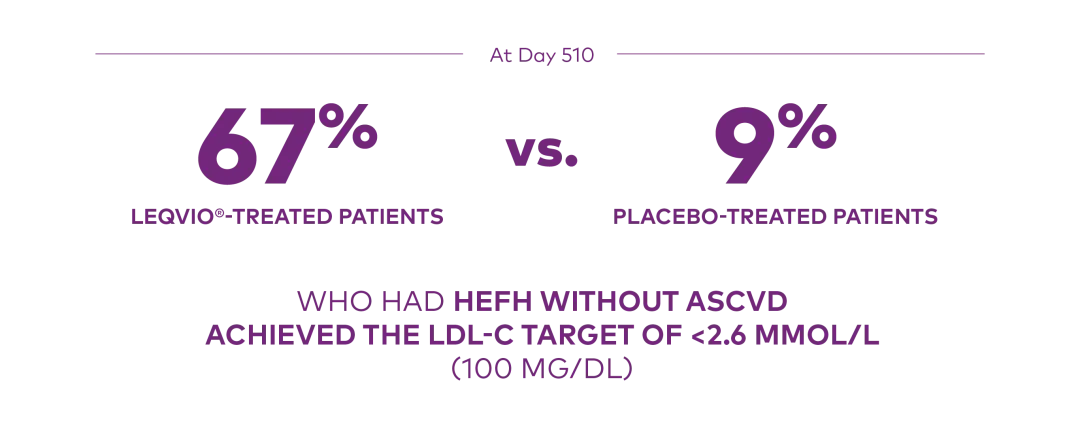
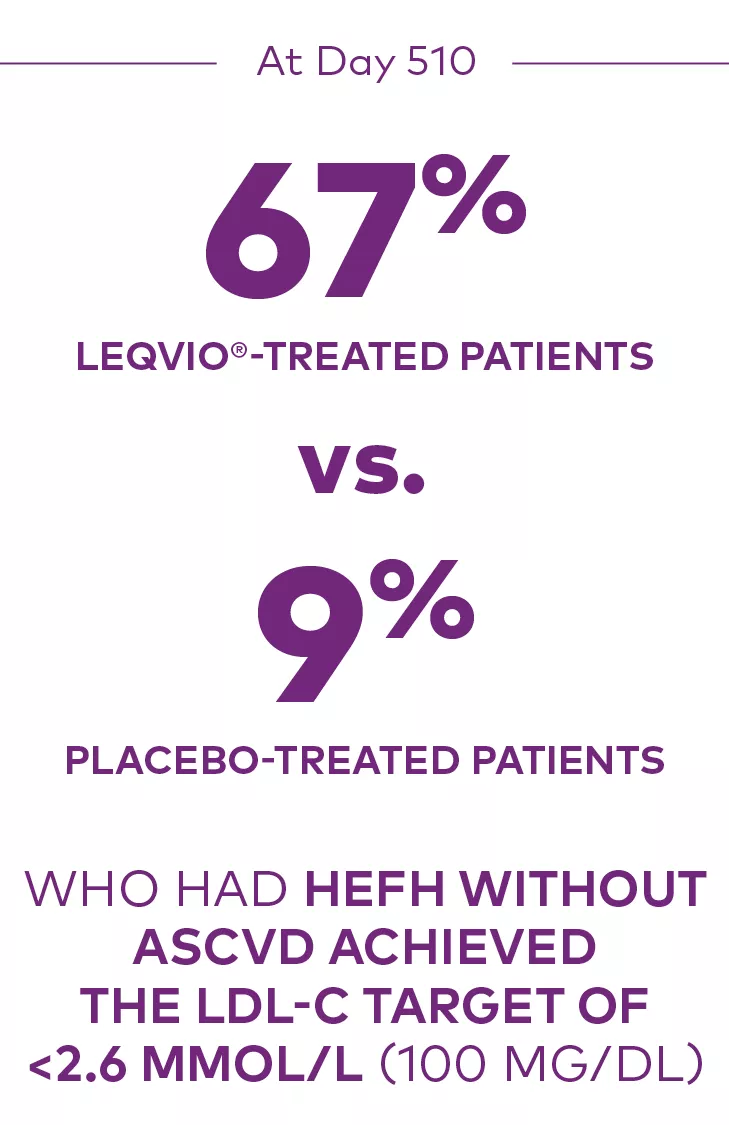
ASCVD=atherosclerotic cardiovascular disease; HeFH=heterozygous familial hypercholesterolemia; LDL-C=low-density lipoprotein cholesterol.
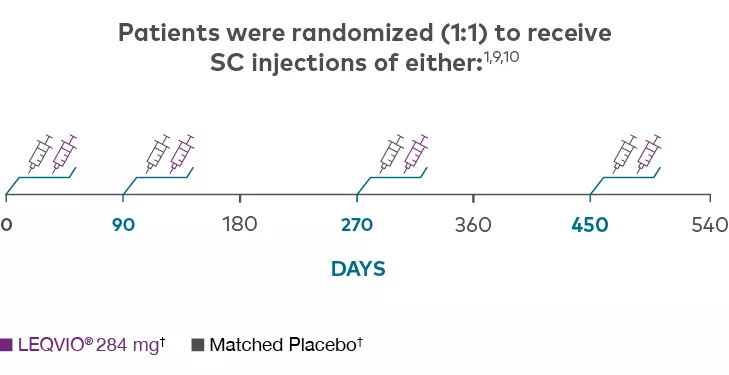
Trial Design
ORION-91,3
Features | Multicentre, double-blind, randomized (1:1), placebo-controlled |
Location | |
Population |
Treatment Arms | LEQVIO® 284 mg SC (n=242) |
Coprimary Endpoints | Percent change in LDL-C from baseline to Day 510 |
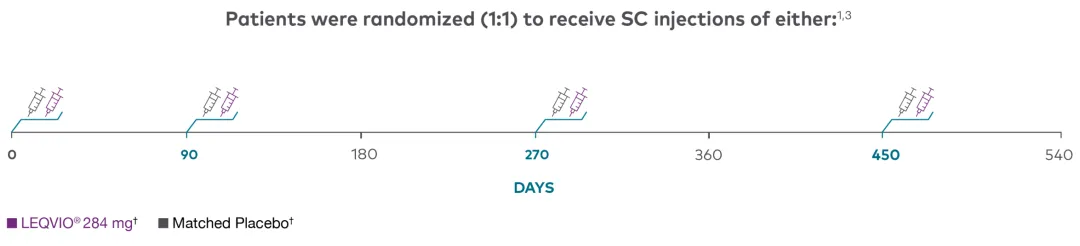
HeFH=heterozygous familial hypercholesterolemia; LDL-C=low-density lipoprotein cholesterol; SC=subcutaneous; WHO=World Health Organization.
† Patients in each study arm in the ORION-9 clinical trial were taking a maximally tolerated dose of a statin, with or without other lipid-modifying therapy, such as ezetimibe.1
References
LEQVIO® is a registered trademark.
© Novartis Pharmaceuticals Canada Inc.
February/2025 - 425307E





