Managing LDL-C
LEQVIO® Clinical Information and Resources
Clinical Experience
Canadian Guidelines
Familial Hypercholesterolemia (FH)
Diagnosing Heterozygous familial hypercholesterolemia (HeFH)
There are several approaches that can help clinicians recognize HeFH in their patients, including:6,7
2021 CCS Dyslipidemia Guidelines
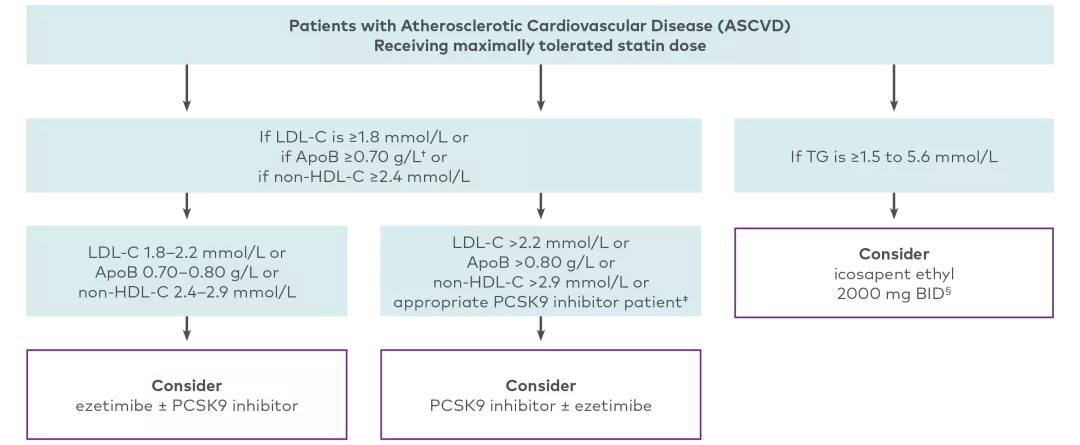
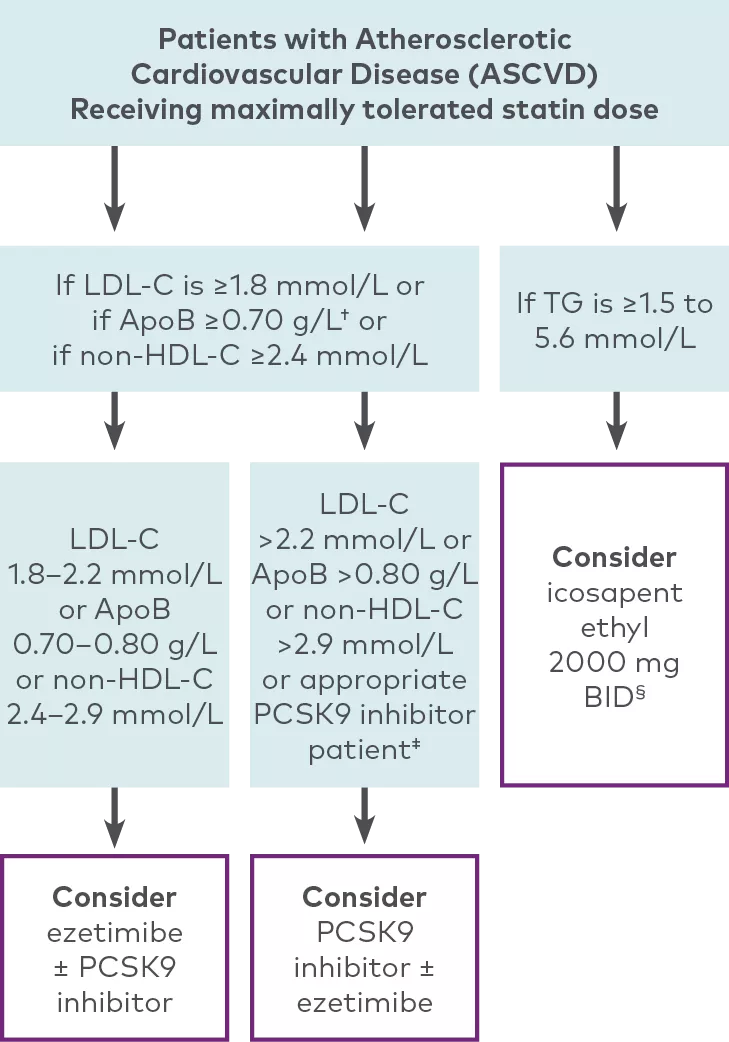
The CCS recommends discussing add-on therapy (ezetimibe or PCSK9 inhibitor) for LDL-C management among patients taking a maximally tolerated dose of a statin and who have:4
HeFH with LDL-C ≥2.5 mmol/L (or <50% reduction), ApoB ≥0.85 g/L, or non-HDL-C ≥3.2mmol/L
ASCVD with LDL-C ≥1.8 mmol/L, ApoB ≥0.70 g/L, or non-HDL-C ≥2.4mmol/L
Patients who may be appropriate for the intensification of lipid-lowering therapy with the addition of a PCSK9 inhibitor4
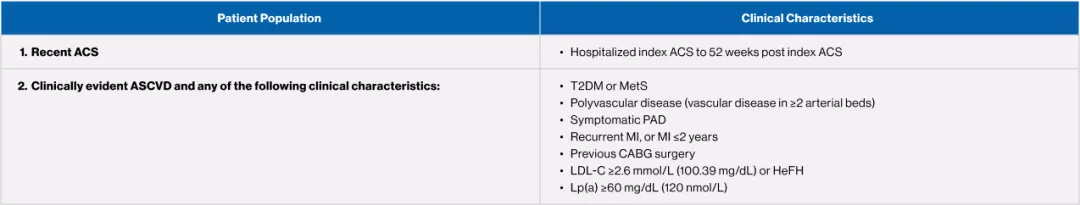
Adapted from the 2021 CCS Dyslipidemia Guidelines Table 3.
The CCS guideline recommendations for PCSK9 inhibitors include evolocumab or alirocumab. The guidelines have not been updated since the market authorization of inclisiran.
References
LEQVIO® Product Monograph. Novartis Pharmaceuticals Canada Inc.
Data on File. Syringe/Needle Size. Novartis Pharmaceuticals Inc., 2023.
Data on File. MOA. Novartis Pharmaceuticals Inc., 2023.
Pearson GJ, Thanassoulis G, Anderson TJ, et al. 2021 Canadian Cardiovascular Society Guidelines for the Management of Dyslipidemia for the Prevention of Cardiovascular Disease in the Adult. Can J Cardiol. 2021;37(8):1129-1150.
Brunham LR, Ruel I, Aljenedil S, et al. Canadian Cardiovascular Society Position Statement on Familial Hypercholesterolemia: Update 2018. Can J Cardiol. 2018;34(12):1553-1563.
Brunham LR, Ruel I, Khoury E, et al. Familial hypercholesterolemia in Canada: Initial results from the FH Canada national registry. Atherosclerosis. 2018;277:419-424.
Ruel I, Brisson D, Aljenedil S, et al. Simplified Canadian Definition for Familial Hypercholesterolemia. Can J Cardiol. 2018;34(9):1210-1214.
LEQVIO® is a registered trademark.
© Novartis Pharmaceuticals Canada Inc.
February/2025 - 425307E