

LEQVIO® Product Monograph. Novartis Pharmaceuticals Canada Inc.
Data on File. First siRNA PCSK9i. Novartis Pharmaceuticals Inc., 2023.
LEQVIO® Product Monograph. Novartis Pharmaceuticals Canada Inc.
Data on File. First siRNA PCSK9i. Novartis Pharmaceuticals Inc., 2023.
Ray KK, Landmesser U, Leiter LA, et al. Inclisiran in Patients at High Cardiovascular Risk with Elevated LDL Cholesterol. N Engl J Med. 2017;376(15):1430-1440.
LEQVIO® Product Monograph. Novartis Pharmaceuticals Canada Inc.
Data On File. First siRNA PCSK9i. Novartis Pharmaceuticals Inc., 2023.
LEQVIO® is a double-stranded siRNA that causes the degradation of PCSK9 mRNA to increase hepatocyte LDL-C receptor recycling and expression.
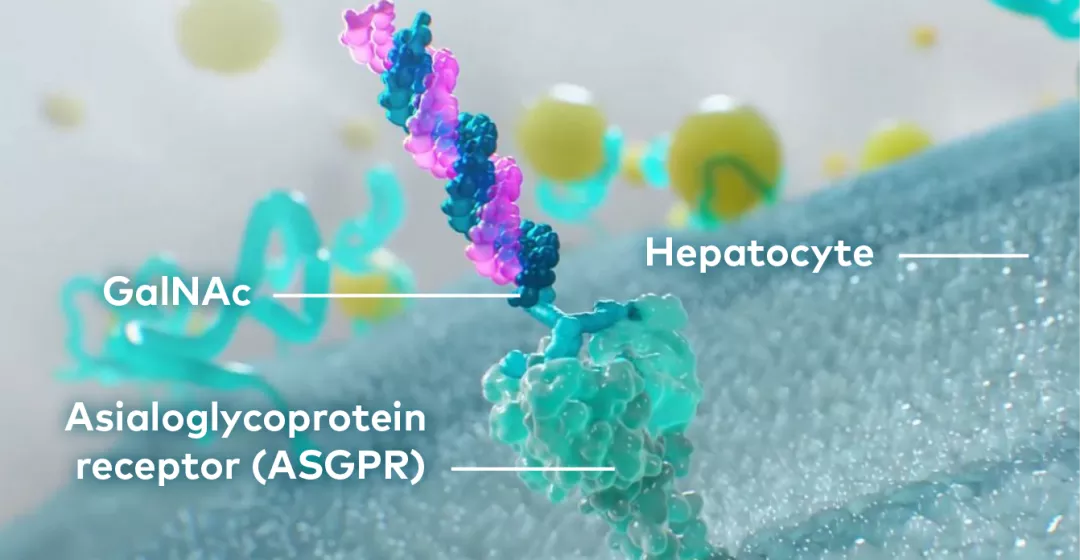
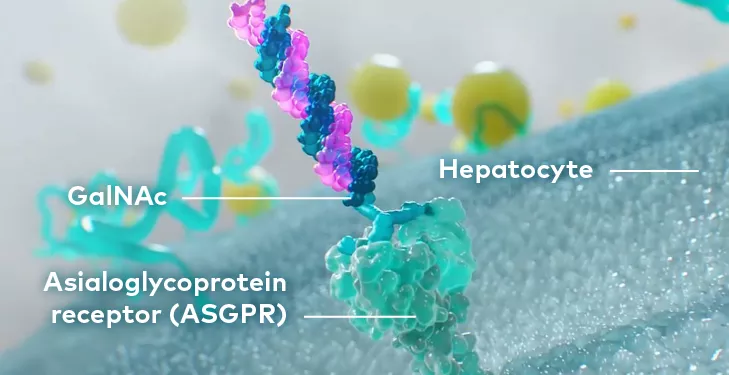
The N‐acetylgalactosamine (GalNAc) conjugated on the sense strand of LEQVIO® facilitates uptake at the liver and selectively targets hepatic ASGPR.
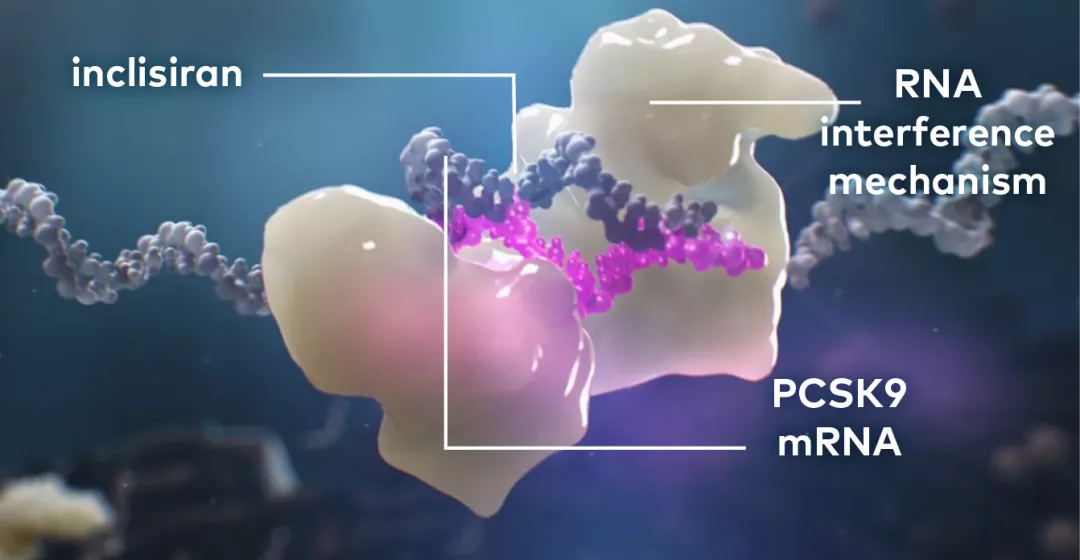
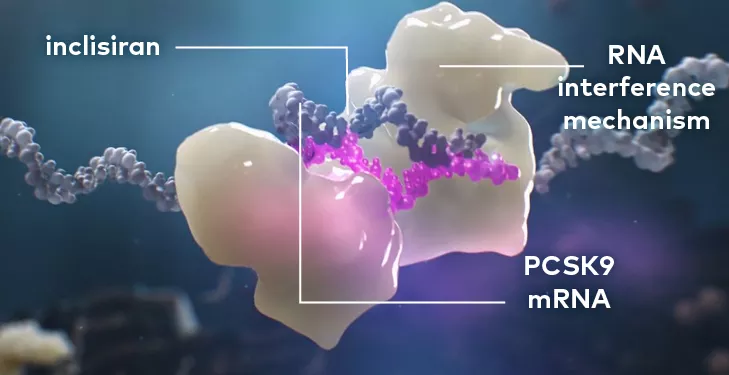
LEQVIO® works with the RNA interference mechanism to direct the catalytic breakdown of mRNA for PCSK9.
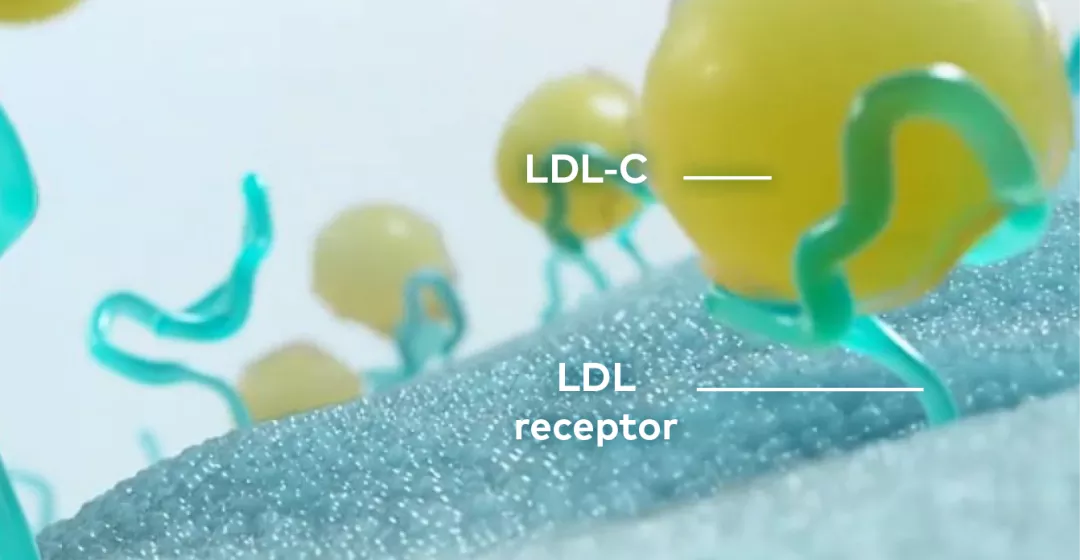
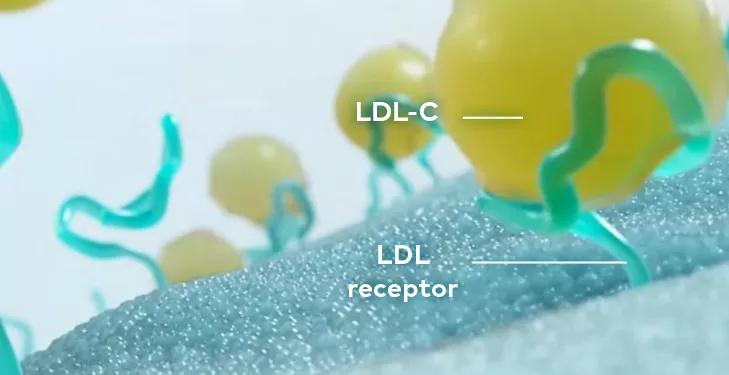
The degradation of PCSK9 mRNA increases LDL receptor recycling and expression on the hepatocyte cell surface, which generally increases LDL-C uptake and lowers LDL-C levels in the circulation.

LEQVIO® (inclisiran injection): Mechanism of Action
 VIDEO
VIDEO
References
LEQVIO® is a registered trademark.
© Novartis Pharmaceuticals Canada Inc.
February/2025 - 425307E

Related content
LEQVIO® Pharmacodynamics†
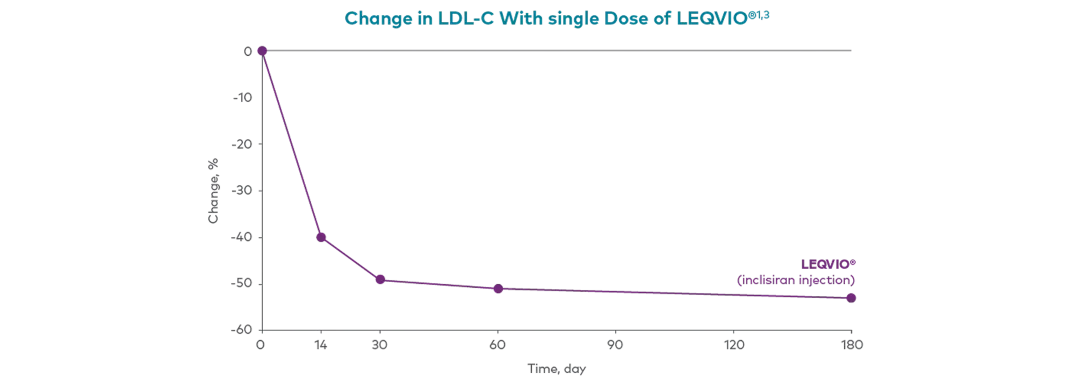
Adapted from Ray, et al., 2017.14
This phase 2 clinical trial provided an overview of percentage change in LDL-C from baseline to multiple timepoints following one (284 mg) or two doses of LEQVIO®1,3
No. Doses (284 mg) | LDL-C reduction post-dose (days)1,3 | |||
14 | 30 | 60 | 180 | |
Single | 40% | 49% | 51% | 38% |
Double | similar to single dose | 53% | ||
LDL-C=low-density lipoprotein cholesterol; PCSK9=proprotein convertase subtilisin/kexin type 9; siRNA=small interfering ribonucleic acid.
† A phase 2, multicentre, double-blind, randomized, placebo-controlled clinical trial of 354 patients with established ASCVD, elevated LDL-C, and who were taking a maximally tolerated dose of a statin. The primary efficacy endpoint was the percent reduction in LDL-C from baseline to Day 180, and was calculated for multiple timepoints at Days 14, 30, 60, 90, 120, 150, 180, 210, and 240.1,3
References
LEQVIO® is a registered trademark.
© Novartis Pharmaceuticals Canada Inc.
February/2025 - 425307E

Related content
References
LEQVIO® is a registered trademark.
© Novartis Pharmaceuticals Canada Inc.
February/2025 - 425307E






