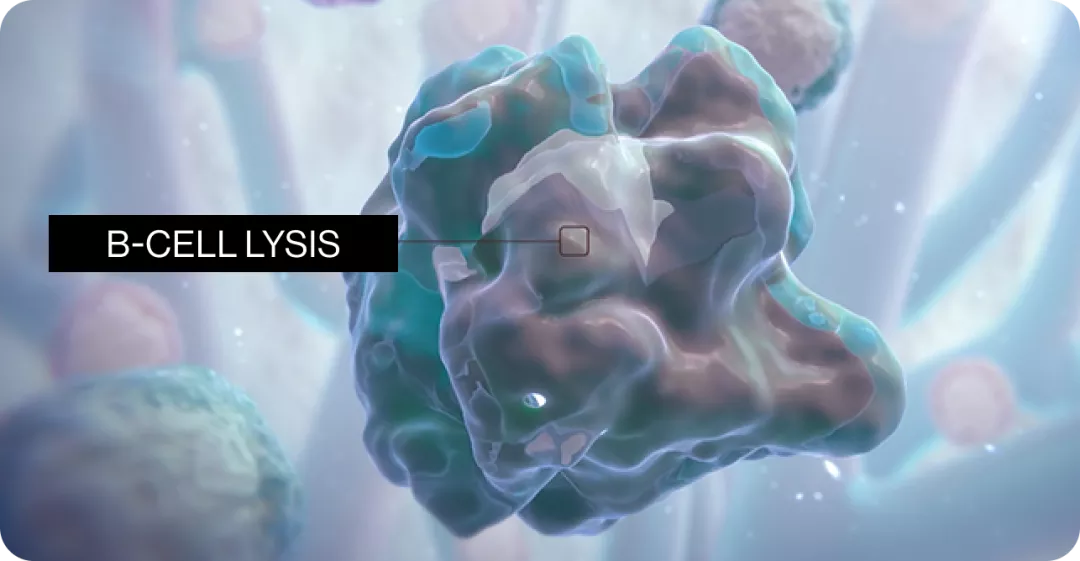
B-cell depletion1‡
|
B-cell repletion1‡
Mechanism of Action
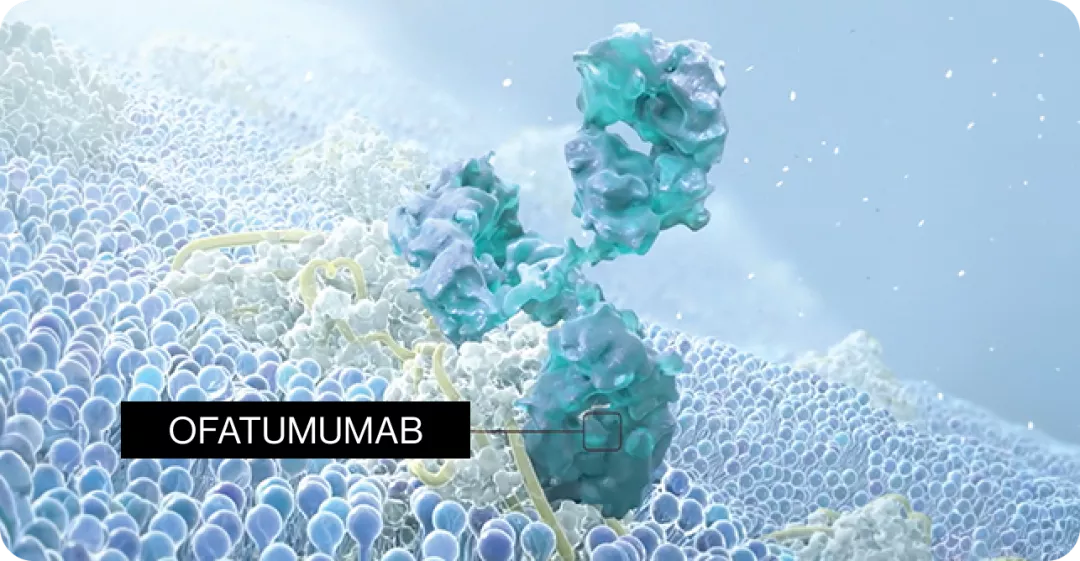
KESIMPTA® binds specifically to the small and large extracellular loops of the CD20+ molecule1✝︎
KESIMPTA® is a selective immunomodulator1✝︎
Ofatumumab is the first and only recombinant fully human monoclonal immunoglobulin G1 (IgG1) antibody against human CD20 expressed on B-cells.1,2* |
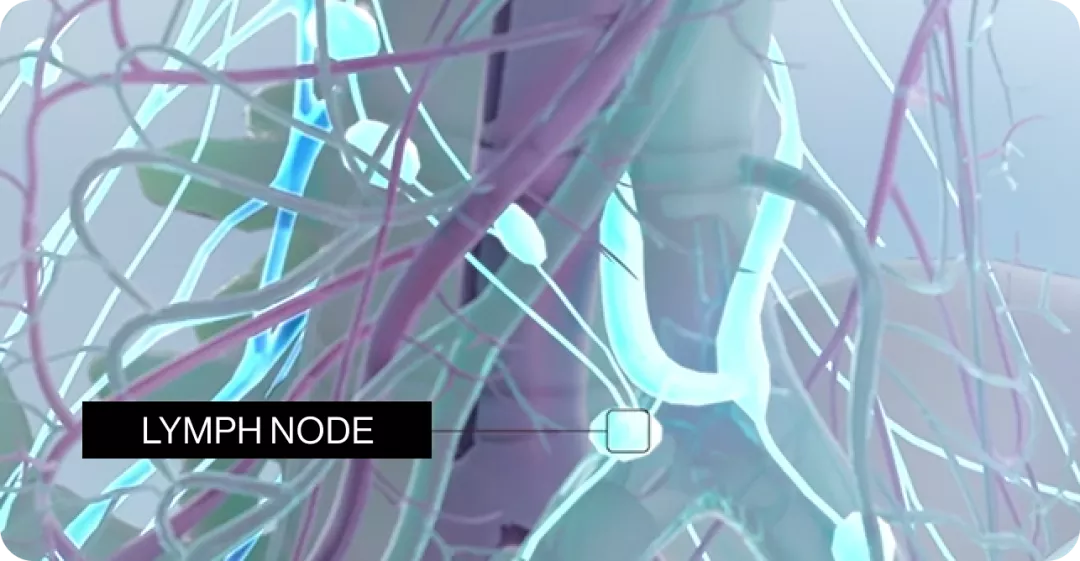
After subcutaneous administration, ofatumumab is believed to be predominantly absorbed via the lymphatic system similarly to other therapeutic monoclonal antibodies.
Images adapted from the KESIMPTA® Product Monograph.
* Comparative clinical significance has not been established.
† Clinical significance has not been established.
‡ Pooled data from treatment epochs of ASCLEPIOS I and II (safety set).
References
KESIMPTA® Product Monograph. Novartis.
Data on file. Novartis Pharmaceuticals Canada Inc.
KESIMPTA, SensoReady and the Go Program are registered trademarks.
© Novartis Pharmaceuticals Canada Inc. September 2025
FA-11447944E