Pre-treatment assessments1
Assessment prior to first dose of KESIMPTA®
Pre-screening
Hepatitis B virus (HBV)
Prior to initiating KESIMPTA®, perform Hepatitis B virus (HBV) screening. KESIMPTA® is contraindicated in patients with active HBV confirmed by positive results for HBsAg and anti-HBV tests.Quantitative serum immunoglobulins
Prior to initiating KESIMPTA®, perform testing for quantitative serum immunoglobulins. For patients with low serum immunoglobulins, consult immunology experts before initiating treatment with KESIMPTA®.
Vaccinations
Because vaccination with live-attenuated or live vaccines is not recommended during treatment and after discontinuation until B-cell repletion, administer all necessary immunizations according to immunization guidelines at least 4 weeks prior to initiation of KESIMPTA® for live or live-attenuated vaccines, and whenever possible, at least 2 weeks prior to initiation of KESIMPTA® for inactivated vaccines.
Assessment before every injection
Infection assessment
In case of active infection, delaying injection of KESIMPTA® should be considered until the infection resolves.Pre-medication
The decision to initiate or continue premedication should be made on an individual patient basis.
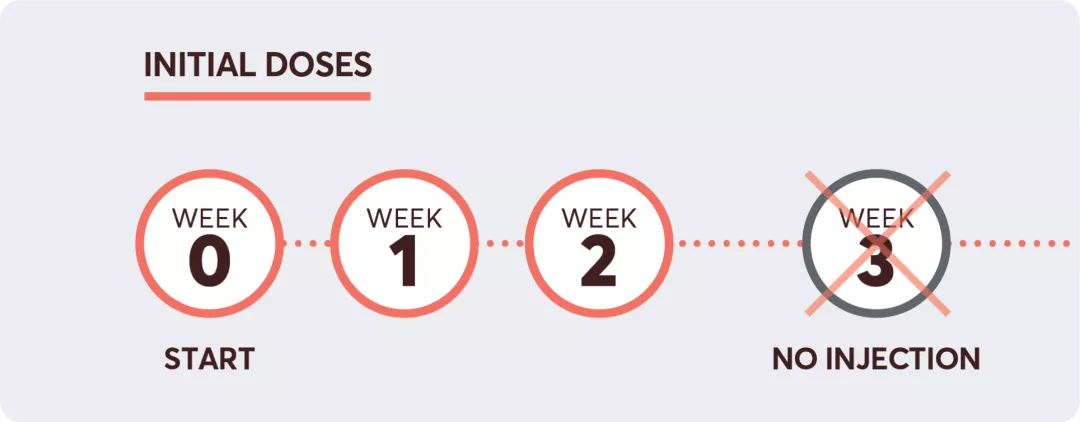
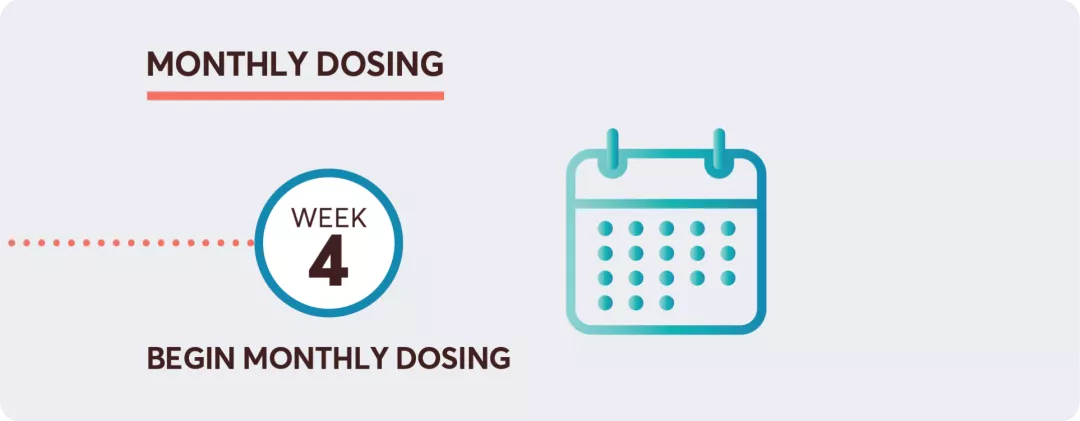
Initiating KESIMPTA®1
Adapted from the KESIMPTA® Product Monograph.
Adapted from the KESIMPTA® Product Monograph.
Please refer to the KESIMPTA® Product Monograph for complete dosing and administration information.
KESIMPTA® is intended for patient self-administration by subcutaneous injection.
The first injection should be performed under the guidance of a healthcare professional.
Missed dose
If an injection of KESIMPTA® is missed, it should be administered as soon as possible without waiting until the next scheduled dose. Subsequent doses should be reset to administer the next sequential dose at the recommended once a month intervals.
Overdosage
Doses up to 700 mg have been administered intravenously in clinical studies with MS patients without dose-limiting toxicity. In the event of overdose, it is recommended that the patient be monitored for any signs or symptoms of adverse reactions and appropriate symptomatic treatment be instituted as necessary.
The flexibility of self-administered injections2
MS=multiple sclerosis; RRMS=relapsing remitting multiple sclerosis.
References
KESIMPTA® Product Monograph. Novartis Pharmaceuticals Canada Inc.
Data on file. Novartis Pharmaceuticals Canada Inc.
KESIMPTA, SensoReady and the Go Program are registered trademarks.
© Novartis Pharmaceuticals Canada Inc. September 2025
FA-11447944E